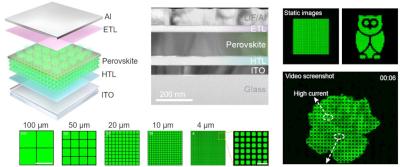
Perovskite LED
What are perovskites?
Perovskite is a calcium titanium oxide mineral, with the chemical formula CaTiO3, discovered in the Ural Mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist Lev Perovski (1792–1856).

Perovskites are a class of materials with a similar structure that are easily synthesized and relatively low-cost. Perovskites are considered the future of solar cells and are also predicted to play a significant role in next-gen electric vehicle batteries, displays, sensors, lasers and much more.
Perovskites can have an impressive collection of interesting properties including “colossal magnetoresistance” - their electrical resistance changes when they are put in a magnetic field (which can be useful for microelectronics). Some Perovskites are superconductors, which means they can conduct electricity with no resistance at all. Perovskite materials exhibit many other interesting and intriguing properties. Ferroelectricity, charge ordering, spin dependent transport, high thermopower and the interplay of structural, magnetic and transport properties are commonly observed features in this family. Perovskites therefore hold exciting opportunities for physicists, chemists and material scientists.
What are LEDs?
A light-emitting diode (LED) is an electronic component that is essentially a two-lead semiconductor light source. It is a p–n junction diode that emits light upon activation by a voltage applied to the leads, which makes electrons recombine with electron holes within the device, releasing energy in the form of photons. This effect is called electroluminescence, and the color of the light is determined by the energy band gap of the chosen semiconductor.
LEDs’ advantages over incandescent light sources include lower energy consumption, longer lifetime, improved physical robustness, smaller size, and faster switching. Light-emitting diodes have become ubiquitous and are found in diverse applications in the aerospace and automotive industries, as well as in advertising, traffic signals, camera flashes and much more.
LEDs meant for general room lighting currently remain more expensive than fluorescent or incandescent sources of similar output, but are significantly more energy efficient.
What can perovskites do for LEDs?
Current high-quality LEDs are based on direct bandgap semiconductors, but making these devices is no easy task because they need to be processed at high temperatures and in vacuum, which makes them rather expensive to produce in large quantities. Perovskites that are direct-bandgap semiconductors could be real alternatives to other types of direct-bandgap materials for applications like color displays, since they are cheap and easy to make and can be easily tuned to emit light of a variety of colors.
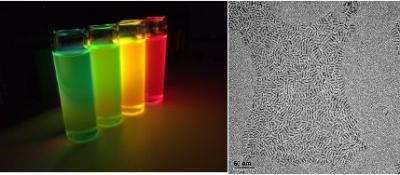
Researchers have found that organometal halide-based perovskites (a combination of lead, organics and halogens that arrange into perovskite crystal structure in the solid state) could be very suitable for making optoelectronics devices, since they can be processed in solution and do not need to be heated to high temperatures. This means that large-area films of these materials can be deposited onto a wide range of flexible or rigid substrates. The perovskites also have an optical bandgap that can be tuned in the visible to infrared regions, which makes them very promising for a range of optoelectronics applications. These materials also emit light very strongly, which makes them very suitable for making LEDs. The light emitted by the perovskites can be easily tuned, which could make them ideal for color displays and lighting, and in optical communication applications.
However, a major obstacle that perovskites will have to overcome in order to be used in LED-type devices is that electrons and holes only weakly bind in perovskite thin films. This means that excitons (electron-hole pairs) spontaneously dissociate into free carriers in the bulk recombination layer, leading to low photoluminescence quantum efficiency (PLQE), high leakage current and low luminous efficiency. This obviously impairs perovskites’ ability to create high-performance LEDs, and for perovskite materials to make a comparable impact in light emission, it is necessary to overcome their slow radiative recombination kinetics. Simply put, researchers will have to find ways of effectively confining electrons and holes in the perovskite so that they can “recombine” to emit light. Major progress is already being made in this field, and it seems that perovskites will indeed open the door to a low-cost, color-tunable approach to LED development.
Perovskite-Info launches a new edition of its Perovskite for Displays Market Report
Perovskite-Info is proud to announce an update to our Perovskite for the Display Industry Market Report. This market report, brought to you by the world's leading perovskite and OLED industry experts, is a comprehensive guide to next-generation perovskite-based solutions for the display industry that enable efficient, low cost and high-quality display devices. The report is now updated to February 2025, with all the latest commercial and research activities.
Reading this report, you'll learn all about:
- Perovskite materials and their properties
- Perovskite applications in the display industry
- Perovskite QDs for color conversion
- Prominent perovskite display related research activities
The report also provides a list of perovskite display companies, datasheets and brochures of pQD film solutions, an introduction to perovskite materials and processes, an introduction to emerging display technologies and more.
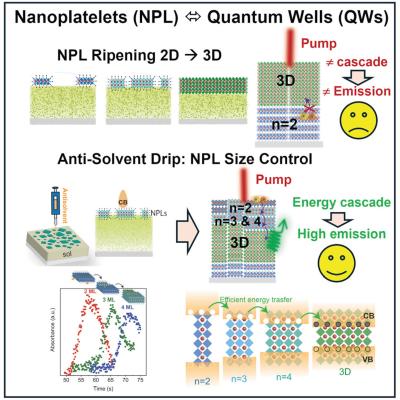
Stable quasi-2D perovskites thin films can enable stable LED applications
While quasi-2D perovskites made with organic spacers co-crystallized with inorganic cesium lead bromide can enable near unity photoluminescence quantum yield at room temperature, LEDs made with such quasi-2D perovskites tend to degrade rapidly - which remains a major bottleneck in this field.
Now, researchers from SUNY University at Buffalo, Texas A&M University, Brookhaven National Laboratory, Missouri University of Science and Technology, National Taiwan University and Yonsei University have shown that the bright emission originates from finely tuned multi-component 2D nano-crystalline phases that are thermodynamically unstable.
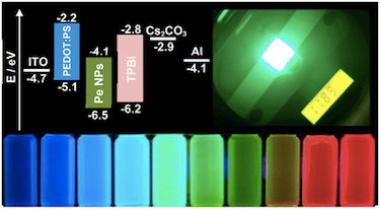
Researchers demonstrate bright perovskite light-emitting diodes by using trifluoroacetate anions in 3D perovskite emitters
Researchers from Sweden's Linköping University, China's Hong Kong Polytechnic University, Northwestern Polytechnical University, Hunan University and Jilin University have demonstrated bright perovskite light-emitting diodes (PeLEDs) with a peak radiance of 2409 W sr−1 m−2 and negligible current-efficiency roll-off, maintaining high external quantum efficiency over 20% even at current densities as high as 2270 mA cm−2.
Device configuration and cross-sectional SEM image of the PeLED. Image from: Nature Communications
One of the key advantages of perovskite light-emitting diodes (PeLEDs) is their potential to achieve high performance at much higher current densities compared to conventional solution-processed emitters. However, state-of-the-art PeLEDs have not yet reached this potential, often suffering from severe current-efficiency roll-off under intensive electrical excitations. The team's new work hopes to help tackle this obstacle.
New method for remote epitaxial growth of perovskite thin films enabled high-resolution micro-LED displays
Miniaturizing LEDs is crucial for creating ultra-high-resolution displays. Metal-halide perovskites show potential for efficient light emission, long-distance carrier transport, and scalable production of bright micro-LED displays. However, current thin-film perovskites face issues with uneven light emission and surface instability during lithography, making them unsuitable for micro-LED devices. There's a strong need for continuous single-crystal perovskite films with minimal grain boundaries, stable surfaces, and uniform optical properties for micro-LEDs. Yet, growing these films and integrating them into devices remains a challenge.
Remote epitaxial crystalline perovskites for ultrahigh resolution micro-LED displays. Image credit: Chinese Academy of Sciences
Recently, researchers from the Chinese Academy of Sciences, University of Science and Technology of China and Jilin University made significant progress in this field. The team developed a novel method for the remote epitaxial growth of continuous crystalline perovskite thin films, that allows for seamless integration into ultrahigh-resolution micro-LEDs with pixels less than 5 μm.
Powering Tomorrow: Solaveni's CEO Discusses Breakthroughs in Green Perovskite Materials
When it comes to innovation in advanced materials, Solaveni GmbH stands out as a company with a bold mission. Founded in 2021 as a subsidiary of Saule Technologies, Solaveni was created with a vision to revolutionize the world of perovskite-based materials by focusing on sustainable chemistry and environmental responsibility. Today, the company is carving out a space in fields like printed electronics, energy harvesting, storage, and solid-state lighting, all while ensuring its processes remain green and future-ready.
At the heart of Solaveni’s journey is its CEO, Dr. Senol Öz, whose expertise and passion for perovskite technology have been key to the company’s progress. Senol’s career spans over a decade of research and hands-on experience in solution-processing and chemical engineering of perovskite solar cells. From his doctoral work in Germany, to his postdoctoral research in Japan, and eventually joining Saule Technologies, his path has been defined by a deep commitment to advancing perovskite materials.
We had the opportunity to sit down with Senol for an insightful Q&A, where he shared his thoughts on Solaveni’s vision, the challenges of perovskite technology, and the future of sustainable material production. Let’s dive into the conversation!
Solaveni was established in 2021 as a subsidiary of Saule Technologies, one of the pioneers in the perovskite solar industry. Why did Saule decide to establish a materials subsidiary?
Saule Technologies, a trailblazer in the perovskite solar industry, founded Solaveni in 2021 to address the burgeoning demand for high-quality, innovative materials critical to advancing solar technology. The establishment of Solaveni reflects Saule’s strategic vision to enhance and diversify its capabilities within the renewable energy sector. By creating a specialized subsidiary, Saule aims to streamline the development and production of materials relevant for the perovskite ecosystem, ensuring consistent quality and fostering innovation.
We interview Sofab Inks' CEO & COO, discussing the company's materials, business, and industry outlook
Sofab Inks develops and produces advanced materials for perovskite solar cells. The company's flagship product is a solvent-based tin oxide ETL that has already seen promising results in improving the performance and lifespan of perovskite solar cells. We interviewed the company's CEO Blake Martin and COO Jack Manzella, who help us understand the company's materials and business better. Click here to contact Sofab Inks to learn more or request a material sample.
Hello Blake and Jack. Earlier this year, Sofab Inks launched Tinfab, a high-performance and low-cost ETL material for perovskite solar cells. Can you detail the market reaction for your new material, and also the performance benefits that one can expect from this new ETL?
Since launching Tinfab, we’ve experienced significant interest across the industry, with approximately 40 companies and universities currently testing the material in perovskite solar cell applications. This strong engagement underscores the market's demand for innovative, scalable ETL solutions.
Tinfab is designed to fully replace C60/fullerenes in perovskite solar cells, addressing key limitations of C60, including lower stability, higher costs, and the complexity of vacuum deposition. Unlike C60, Tinfab can be solution-deposited in ambient environments, making it far more suitable for scalable manufacturing.
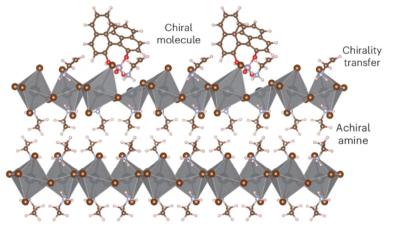
New process induces chirality in halide perovskite semiconductors
Researchers at the U.S. Department of Energy’s National Renewable Energy Laboratory (NREL) and the Center for Hybrid Organic Inorganic Semiconductors for Energy (CHOISE), an Energy Frontier Research Center (EFRC), University of Wisconsin-Madison, University of Colorado Boulder, Duke University and University of Utah have discovered a new process to induce chirality in halide perovskite semiconductors, which could open the door to cutting-edge electronic applications.
The development is the latest in a series of advancements made by the team involving the introduction and control of chirality. Chirality refers to a structure that cannot be superimposed on its mirror image, such as a hand, and allows greater control of electrons by directing their “spin.” Most traditional optoelectronic devices in use today exploit control of charge and light but not the spin of the electron.
New method enables researchers to engineer layered perovskite materials at the atomic level
Researchers at North Carolina State University and Brookhaven National Laboratory have reported a technique for engineering layered hybrid perovskites (LHPs) down to the atomic level, which enables precise control on how the materials convert electrical charge into light.
Image credit: Matter
The technique opens the door to engineering materials tailored for use in next-generation printed LEDs, lasers and photovoltaic devices.
Researches develop novel method to achieve efficient and stable blue perovskite LEDs
Soochow University researchers have proposed the in situ treatment of Cl-rich benzene phosphorus oxydichloride (BPOD)as a way to achieve high-quality pure-blue perovskites, by simultaneously enlarging the perovskite bandgap, passivating the halide vacancy defects, and immobilizing the halide ions through the hydrolysis products of chloride ions and phenylphosphonic acid.
The background for this work is that despite the substantial progress in sky-blue (480−495 nm) perovskite light-emitting diodes (PeLEDs), pure-blue PeLEDs (<480 nm) merely show moderate performances. Bromide-chloride mixed perovskites may have potential to enable a straightforward and effective way to obtain pure-blue emission, but the tricky issue of halide migration in mixed halide perovskites makes it challenging to achieve efficient PeLEDs with stable electroluminescence (EL) spectra.
Novel approach enables simple and efficient perovskite LEDs with record brightness
A team of researchers, led by Professor David Di from the International College of Zhejiang University and the School of Optoelectronic Science and Engineering, recently achieved a continuous transition from n-type to p-type perovskite semiconductors through molecular doping, while maintaining extremely high luminescence performance.
Image credit: Zhejiang University
Based on controllable doping, the team developed a perovskite LED with a simple structure and reported a record for the highest brightness of solution-based LEDs, reaching 1.16 million nits.
Pagination
- Page 1
- Next page