Scientists at the National Renewable Energy Laboratory (NREL) have investigated how to manufacture perovskite materials and solar technology with human health in mind.
Newly published research points to the safest choices for solvents needed to make perovskite solar cells. Unlike silicon solar panels, which require an industrial process, perovskite solar panels can be made using chemicals and produced using a roll-to-roll printing method or spray coating. The process also costs less and takes less time than manufacturing silicon panels.
'We wanted to predict which solvents could have the biggest issues from toxicity data,' Luther said. 'If certain solvents were very problematic, we want to know that now, while the stage of perovskite solar cell research is still in the development phase.'
The researchers concluded that the risk to human health from solvents used to make perovskites was surprisingly low compared to some other industrial processes now in use but desired a better understanding of the potential dangers.
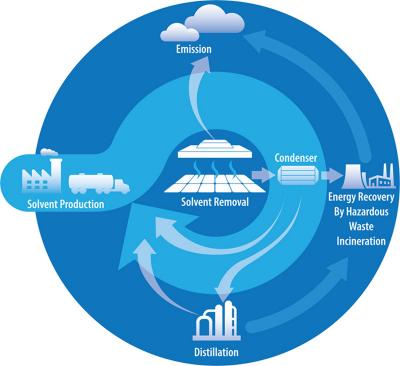
The use of lead in perovskites has spurred many discussions, but the use of solvents at an industrial scale has not been analyzed in depth. Solvents are used to help dissolve the precursor salts and play a role in the crystallization of the perovskites, and certain solvents have been shown to produce more efficient solar cells. A large-scale factory able to produce 1 gigawatt worth of perovskite solar panels per year'the equivalent of 3'4 million panels'would require about 3,500 liters of solvent.
In a commercial process, after the solvent is used, it is then either discharged to the environment or collected for recycling or incineration.
In the latest research, the scientists studied the human health toxicity and other environmental impacts associated with the use of eight solvents that have been used in the production of perovskites. The most commonly used solvent in perovskites is dimethylformamide (DMF), which is recognized as toxic to the human reproductive system.
To analyze the eight solvents, the researchers used a variety of existing databases of toxicity data, and they considered the production of each solvent as well. The researchers also considered what would be done with the solvents after processing the perovskite active layers: discharged, burned, or recovered for recycling. For all solvents considered, recovery was deemed to the most environmentally friendly despite requiring additional energy to recover and recycle.
The researchers determined dimethylsulfoxide (DMSO) poses the lowest risk to the environment or to human health out of the solvents considered but pointed out perovskite solar cells produced using DMSO are not the most efficient at this time.
The scientists said their research should be considered a roadmap for the further development of less hazardous solvents. They noted that the total amount of solvent used at the industrial scale would be marginal and 'a significant impact on human health due to solvent use is not likely.' But they cautioned future legislation could limit the large-scale industrial use of certain solvents, 'which is why we encourage further development of greener processes.'



