Tokyo Institute of Technology researchers have shown that donor doping into a mother material with disordered intrinsic oxygen vacancies, instead of the widely used strategy of acceptor doping into a material without oxygen vacancies, can greatly enhance the conductivity and stability of perovskite-type proton conductors at intermediate and low temperatures of 250–400 ℃, (e.g. 10 mS/cm at 320 ℃). This approach provides a new design direction for proton conductors for fuel cells and electrolysis cells.
Protonic ceramic (or proton conducting) fuel/electrolysis cells (PCFCs/PCECs) are a strong contender for future sustainable energy technologies. These devices can directly convert chemical energy into electricity and vice versa with zero emissions at low or intermediate temperatures, making them an attractive option for many emerging applications such as next-generation distributed power sources. In addition, unlike other types of fuel cells and electrolysers, the PCFCs/PCECs do not require precious metal catalysts or expensive, heat-resistant alloys.
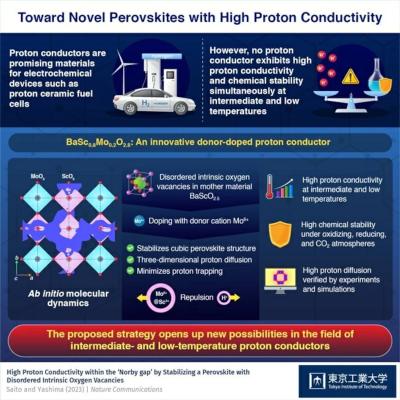
However, there have been no reports of proton conductors with both high conductivity as well as high stability at intermediate and low temperatures of 250–400 ℃. This problem is known as the "Norby gap," and scientists have been searching for materials that can overcome it for many years.
This issue lead Professor Masatomo Yashima and Mr. Kei Saito from Tokyo Institute of Technology (Tokyo Tech) in Japan to propose a new strategy that could revolutionize the design and development of proton conductors.
The researchers tackled one of the main drawbacks of state-of-the-art perovskite-type proton conductors. These materials have the formula A2+B4+O3, where A and B are larger and smaller cations, respectively. A general strategy to enhance the proton conductivity in such perovskites is to introduce an acceptor dopant; that is, a cation M3+ with a valence lower than that of B4+. These "impurities" create oxygen vacancies in the resulting crystalline lattice, which, in turn, increases proton conductivity. However, this approach also creates a problem known as "proton trapping," whereby protons are trapped by the acceptor dopant M3+, which has an effective negative charge relative to the host cation B4+, due to the electrostatic attraction.
To avoid this issue, the researchers turned to BaScO2.5. This perovskite has intrinsic (or inherent) oxygen vacancies in its crystal structure, which enables donor doping. The team doped donor dopant Mo6+ into BaScO2.5 to produce BaSc0.8Mo0.2O2.8 (or "BSM20"). "Contrary to the conventional acceptor doping approach, donor doping can reduce proton trapping effect through the electrostatic repulsion between protons and the donor Mo6+ cations, which have a higher valence than the host cation Sc3+," explains Prof. Yashima. "This, in turn, leads to high proton conduction."
Following a series of experiments and theoretical analyses using advanced simulation techniques, the researchers demonstrated that BSM20 indeed offered exceptionally high proton conductivity at intermediate and low temperatures in the Norby gap. Moreover, donor doping helped stabilize a cubic perovskite-type structure, enabling efficient three-dimensional proton conduction throughout the material. Notably, BSM20 also exhibited remarkably high stability under oxidizing, reducing, and carbon dioxide atmospheres, a property essential for many practical applications.
Overall, the findings of this study could pave the way for new proton conductors for PCFCs/PCECs with unprecedented performance. "The proposed strategies and the discovery of BSM20 could have a significant impact on energy and environmental science and technology," concludes Prof. Yashima.