Researchers from Stanford University and Mississippi State University recently explored the potential of Mn2+-doped perovskite LEDs (PeLEDs) for lighting and display applications.
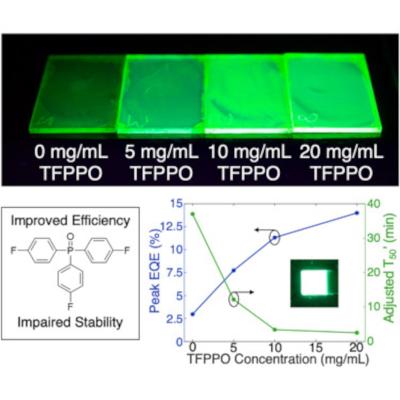
By introducing a molecular additive, tris(4-fluorphenyl)phosphine oxide (TFPPO), Mn2+-doped PeLEDs achieved a peak external quantum efficiency of 14.0% and peak luminance (i.e., brightness) of 128,000 cd/m2. These high efficiencies and brightnesses suggest that Mn2+-doped PeLEDs could be implemented in lighting or display applications. However, device stability is also important to consider. The team found that introducing TFPPO compromises the stability of Mn2+-doped PeLEDs—a decrease from 37.0 to 2.54 min. By analyzing both the optoelectronic and photophysical characteristics of Mn2+-doped PeLEDs before and after device operation, the scientists reported insights into this efficiency-stability trade-off.
This method can boost the brightness and efficiency of PeLEDs, a cheaper and easier-to-make alternative than current LEDs. These enhancements, however, caused the lights to fizzle out within minutes, demonstrating the careful trade-offs that must be understood to advance this class of materials.
“We took some big steps towards understanding why it’s degrading. The question is, can we find a way to mitigate that while keeping the efficiency?” says Dan Congreve, assistant professor of electrical engineering and senior author on the paper. “If we can do that, I think we can really start to work towards a viable commercial solution.”
In simple terms, LEDs transform electrical energy into light by passing electric current through a semiconductor – layers of crystalline material that emits light with an applied electric field. But creating those semiconductors gets complex and costly compared to less energy-efficient lights like incandescents and fluorescents.
“A lot of these materials are grown on expensive surfaces such as a four-inch sapphire substrate,” says Sebastian Fernández, a PhD student in Congreve’s lab and the paper’s lead author. “Just to purchase this substrate costs a few hundred dollars.”
PeLEDs use metal halide perovskites, composed of a blend of different elements. Engineers can grow perovskite crystals on glass substrates, saving a significant sum compared to normal LEDs. They can also dissolve perovskites in solution and “paint” it onto glass to create a light-emitting layer, a simpler production process than regular LEDs call for.
These advantages could make energy-efficient indoor lighting feasible for more of the built environment, reducing energy demand. PeLEDs could also sharpen the color purity of smartphone and TV displays. “A green is more green, a blue is more blue,” Congreve says. “You can literally see more colors from the device.”
Most PeLEDs today, however, degrade after just a few hours. And they often don’t match the energy efficiency of standard LEDs, due to random gaps in the perovskite’s atomic structure known as defects. “There should be an atom here, but there’s not,” Congreve explains. “Energy goes in there, but you don’t get light out, so it harms the overall efficiency of the device.”
To mitigate these issues, Fernández built on a technique debuted by Congreve and Mahesh Gangishetty, assistant professor of chemistry at Mississippi State University and a co-author on the paper. Many of those energy-wasting gaps in perovskites occur where atoms of lead ought to be. By replacing 30% of the perovskite’s lead with manganese atoms, which helps fill those gaps, the team more than doubled their PeLEDs’ brightness, almost tripled efficiency, and extended the lights’ lifespans from less than one minute to 37 minutes.
The technique also has the potential to mitigate potential health risks. “Lead is extremely important for light emission within this material, but at the same time, lead is known to be toxic,” Fernández says. This type of lead is also water-soluble – meaning it could leak through, say, a cracked smartphone screen. “People are skeptical of commercial technology that is toxic, so that also pushed me to consider other materials.”
But Fernández went one step further, mixing a phosphine oxide called TFPPO into the perovskite. “I added it and saw the efficiencies just shoot up,” he says. The additive made the lights up to five times more energy-efficient than those with only a manganese boost and brought out one of the brightest glows of any PeLED yet recorded.
But the gains were not without a downside: the lights faded to half their peak brightness in just two and a half minutes. (On the other hand, the perovskites that weren’t treated with TFPPO are the version that sustained their brightness for 37 minutes.)
Fernández thinks that the transformation of electrical energy into light over time in PeLEDs with TFPPO becomes less efficient than in those without, largely due to increased obstacles related to charge transport within the PeLED. The team also suggests that while TFPPO initially fills some gaps in the perovskite’s atomic structure, those gaps quickly reopen, causing energy efficiency to drop off along with durability.
Moving forward, Fernández hopes to experiment with different phosphine oxide additives to see whether they yield different effects, and why.
“Clearly, this additive is incredible in terms of efficiency,” Fernández says. “However, its effects on stability need to be suppressed to have any hope to commercialize this material.”
Congreve’s lab is working to address other limitations of PeLEDs, too, such as their difficulty with producing violet and ultraviolet light. In another recent paper in the journal Matter, led by PhD student Manchen Hu, the team found that by adding water to the solution in which the perovskite crystals form, they could produce PeLEDs that emitted bright violet light five times more efficiently. With further improvements, ultraviolet PeLEDs could sterilize medical equipment, purify water, and help grow indoor crops – all more affordably than current LEDs allow.