Researchers from the U.S. Department of Energy's (DoE) Argonne National Laboratory, Brookhaven National Laboratory, Los Alamos National Laboratory, SLAC National Accelerator Laboratory and Taiwan's Academia Sinica have reported the preparation of stable perovskite nanocrystals for LEDs.
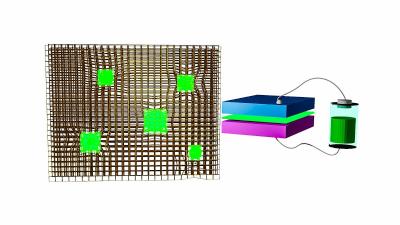 Light-emitting diodes made from perovskite nanocrystals (green) embedded in a metal-organic framework. Image from Phys.org
Light-emitting diodes made from perovskite nanocrystals (green) embedded in a metal-organic framework. Image from Phys.org
Perovskite nanocrystals' unstable nature has so far hindered their potential to be used as LED materials. However, the research team managed to stabilize the nanocrystals in a porous structure called a metal-organic framework, or MOF for short. Based on earth-abundant materials and fabricated at room temperature, these LEDs could one day enable lower cost TVs and consumer electronics, as well as better gamma-ray imaging devices and even self-powered X-ray detectors with applications in medicine, security scanning and scientific research.
Hsinhan Tsai, a former J. R. Oppenheimer postdoc fellow at Los Alamos, added, "The intriguing concept of combining perovskite nanocrystal in MOF had been demonstrated in powder form, but this is the first time we successfully integrated it as the emission layer in an LED."
Previous attempts to create nanocrystal LEDs were thwarted by the nanocrystals degrading back to the unwanted bulk phase, losing their nanocrystal advantages and undermining their potential as practical LEDs. Bulk materials consist of billions of atoms. Materials such as perovskites in the nano phase are made of groupings of just a few to a few thousand atoms, and thus behave differently.
In their novel approach, the research team stabilized the nanocrystals by fabricating them within the matrix of a MOF, like tennis balls caught in a chain-link fence. They used lead nodes in the framework as the metal precursor and halide salts as the organic material. The solution of halide salts contains methylammonium bromide, which reacts with lead in the framework to assemble nanocrystals around the lead core trapped in the matrix. The matrix keeps the nanocrystals separated, so they don't interact and degrade. This method is based on a solution coating approach, far less expensive than the vacuum processing used to create the inorganic LEDs in wide use today.
The MOF-stabilized LEDs can be fabricated to create bright red, blue and green light, along with varying shades of each.
"In this work, we demonstrated for the first time that perovskite nanocrystals stabilized in a MOF will create bright, stable LEDs in a range of colors," said Wanyi Nie, scientist in the Center for Integrated Nanotechnologies at Los Alamos National Laboratory. "We can create different colors, improve color purity and increase photoluminescence quantum yield, which is a measure of a material's ability to produce light."
The research team used the Advanced Photon Source (APS), a DoE Office of Science User Facility at Argonne, to perform time-resolved X-ray absorption spectroscopy, a technique that allowed them to spot the changes in the perovskite material over time. Researchers were able to track electrical charges as they moved through the material and learned important information about what happens when light is emitted.
"We could only do this with the powerful single X-ray pulses and unique timing structure of the APS," said Xiaoyi Zhang, group leader with Argonne's X-ray Science Division. "We can follow where the charged particles were located inside the tiny perovskite crystals."
In durability tests, the material performed well under ultraviolet radiation, in heat and in an electrical field without degrading and losing its light-detecting and light-emitting efficiency, a key condition for practical applications such as TVs and radiation detectors.



